Is Used to Describe a Liquid That Evaporates Easily
Heat from the sun or solar energy powers the evaporation process. Describe the general shape of a liquid and use KMT to explain.
Which Is The Slower Process Evaporation Or Boiling Quora
The magnitude of temperature decrease is related to the strength of intermolecular forces of attraction.
. It can be easily visualized when rain puddles disappear on a hot day or when wet clothes dry in the sun. Sweat evaporates due to its liquid characteristic. If so you may have used rubbing alcohol to help cool your skin.
A liquid can evaporate at any temperature producing vapor above the surface of the liquid. When this pressure equals. A puddle of water will evaporate much faster from the hot pavement than it would from a cool kitchen floor.
The pressure from the vapor of a liquid as it evaporates depends on temp liquid molecules need enough speed to overcome attraction of other molecules evaporating liquids____ heat from its surroundings. The evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. If the solution was say alcohol water then as both have a large vapour pressure at room temperature both will evaporate and nothing will be left.
For example diethyl ether is a nonpolar liquid with weak dispersion forces. Its vapor pressure at 20C is 5896 kPa. Acetone CH3COCH3 is a laboratory solvent that is also commonly used as a.
Evaporation is a very important part of the water cycle. The gaseous state is sometimes called the vapour phase which forms when a liquid evaporates. This vapor exerts a pressure which is called the _____ pressure.
Identify three websites you will use to start your research. If you use other websites to complete the research questions in Part 2 add them to this list. A volatile liquid one that easily evaporates is put into a jar and the jar is then sealed.
Do all of the acetone molecules moving away from the surface of the liquid escape. The sweat of our body evaporates taking the heat. As with puddles when some liquid is spilled on the floor or cleaned the heat causes this liquid to evaporate completely dry.
If not why not. What three criteria must be met for a. Research 20 points Use the websites you listed in Part 1 to answer the following questions.
The water solvent evaporates due to the difference in vapour pressure at the liquid surface and the average value in a room. When evaporating the rate of evaporation increases with temperature and the rate of removing. When water is heated it evaporates.
Vocabulary Evaporation happens when a liquid turns into a gas. All liquids evaporate except when in a closed container then no liquids evaporate. 7- Evaporation of Nail Paint Remover.
The only difference is the rate at. Liquids which evaporate easily have high vapor pressure. What is a liquid that evaporates easily called.
A liquid with stronger intermolecular forces does not evaporate easily and thus has a lower vapor pressure. Describe the submicroscopic events taking place at the surface of liquid acetone when it evaporates. Using KMT explain the evaporation rate of a volatile liquid.
The alcohol works because it evaporates quickly and lowers skin temperature. - Answers In chemistry thermodynamics and other areas we use the term volatility to speak to the characteric of a substance to easily vaporize. Describe the evaporation of a liquid and its relationship to the kinetic energy of the evaporating particles.
5- Evaporation of the transpiration of our body. Cross out any websites that dont end up helping you complete the activity. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor.
This pressure _____ as the temperature of the liquid increases. 3 points Part 2. The higher the temperature of the liquid the faster it will evaporate.
This is why the gaseous state of water is sometimes called water vapour. In these examples the liquid water is not actually vanishingit is evaporating into a gas called water vapor. It soaks up moisture from.
Describe a liquid in terms of particle spacing. The same goes for acetone and water. When heated the particles of the liquid move faster allowing the liquid to flow more easily.
Slow evaporation rate Has strong IM forces between particles so it requires MORE kinetic energy to become a. Evaporation can generally be defined as a process by which a liquid or solid is transformed into vapour. Evaporation happens when a liquid substance becomes a gas.
The dye is chemically unchanged by not being dissolved in a solvent. This is an effect of evaporation or the change of matter from its liquid state to its vapour state. Evaporation Because the particles of a.
A volatile liquid one that easily bartleby. 6- Drying the wet floor. When you spray perfume on your body your body feels slightly cooler.
Evaporation happens on a global scale.

Intermolecular Forces Demonstration Relative Evaporation Rates Of Volatile Liquids Chemdemos
Which Liquid Evaporates The Fastest By Alexander Ostolaza

Evaporation Chemistry For Non Majors
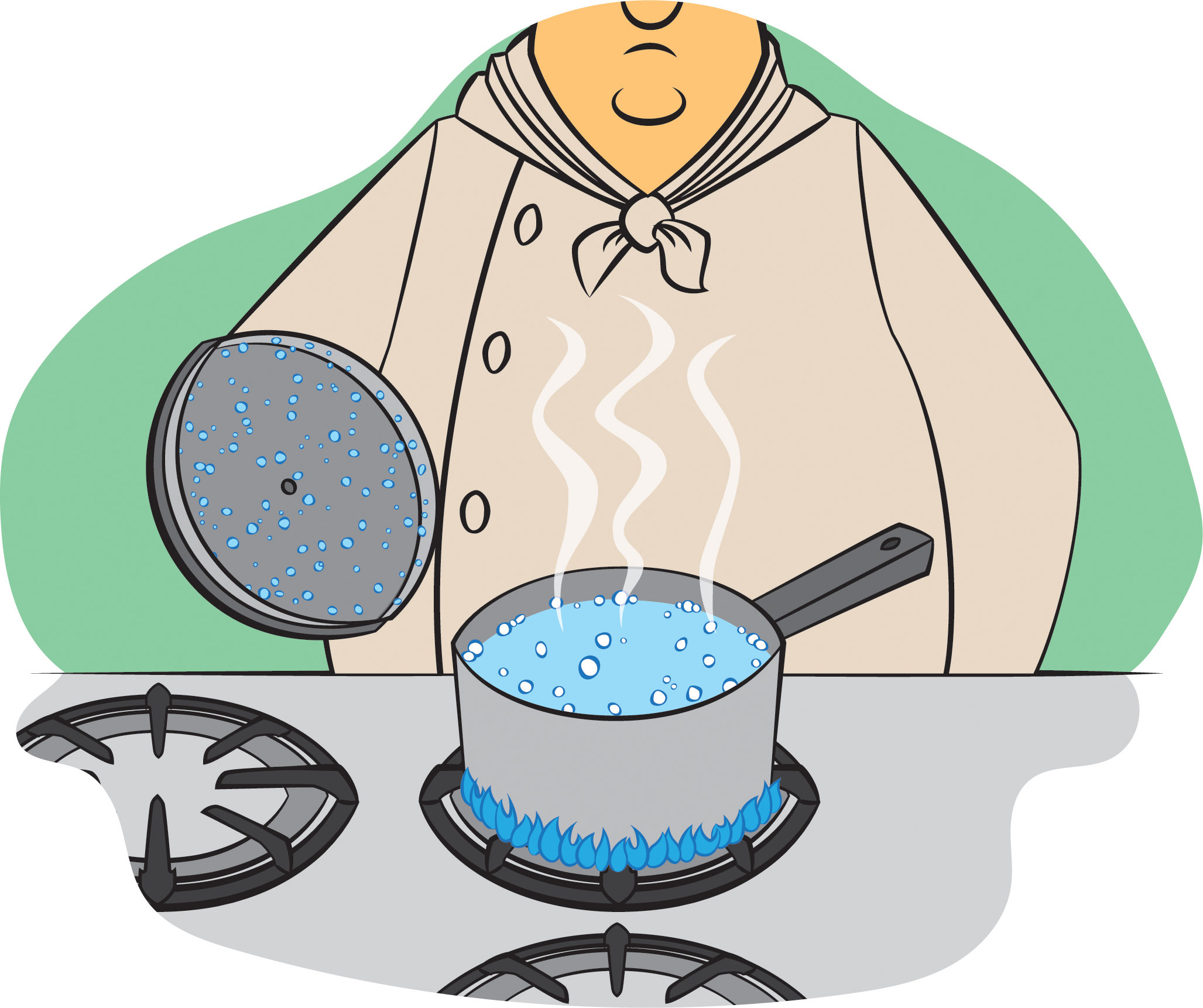
Q What S The Difference Between Evaporation And Boiling Nsta
Chilling Science Evaporative Cooling With Liquids Scientific American

What Is Evaporation Definition Facts And Examples Twinkl

What Liquids Evaporate Faster Lisbdnet Com
Why Do Liquids Evaporate When They Aren T At Their Boiling Temperature Quora
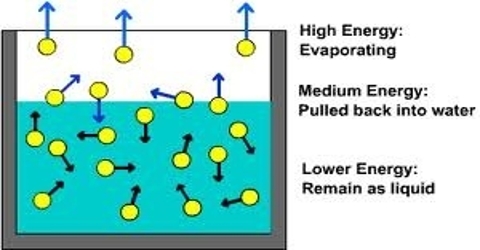
Rapid Evaporation Of Volatile Liquids Qs Study
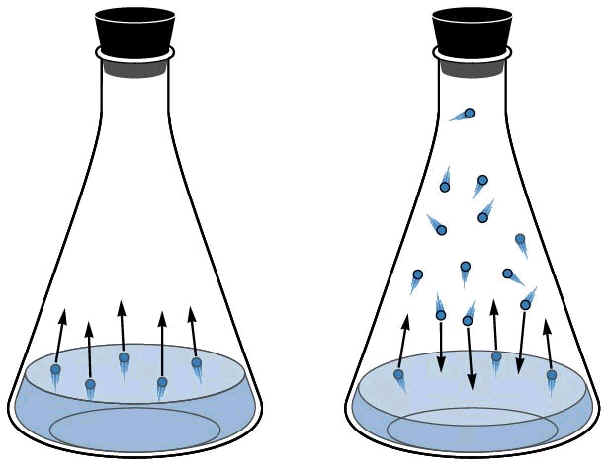
Everyday Chemistry What Happens To The Molecules Of A Liquid When It Evaporates Chemistry Stack Exchange

Separation By Evaporation Geeksforgeeks
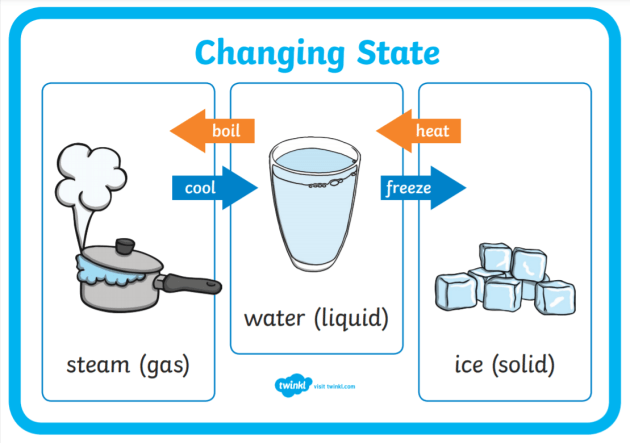
What Is Evaporation Definition Facts And Examples Twinkl
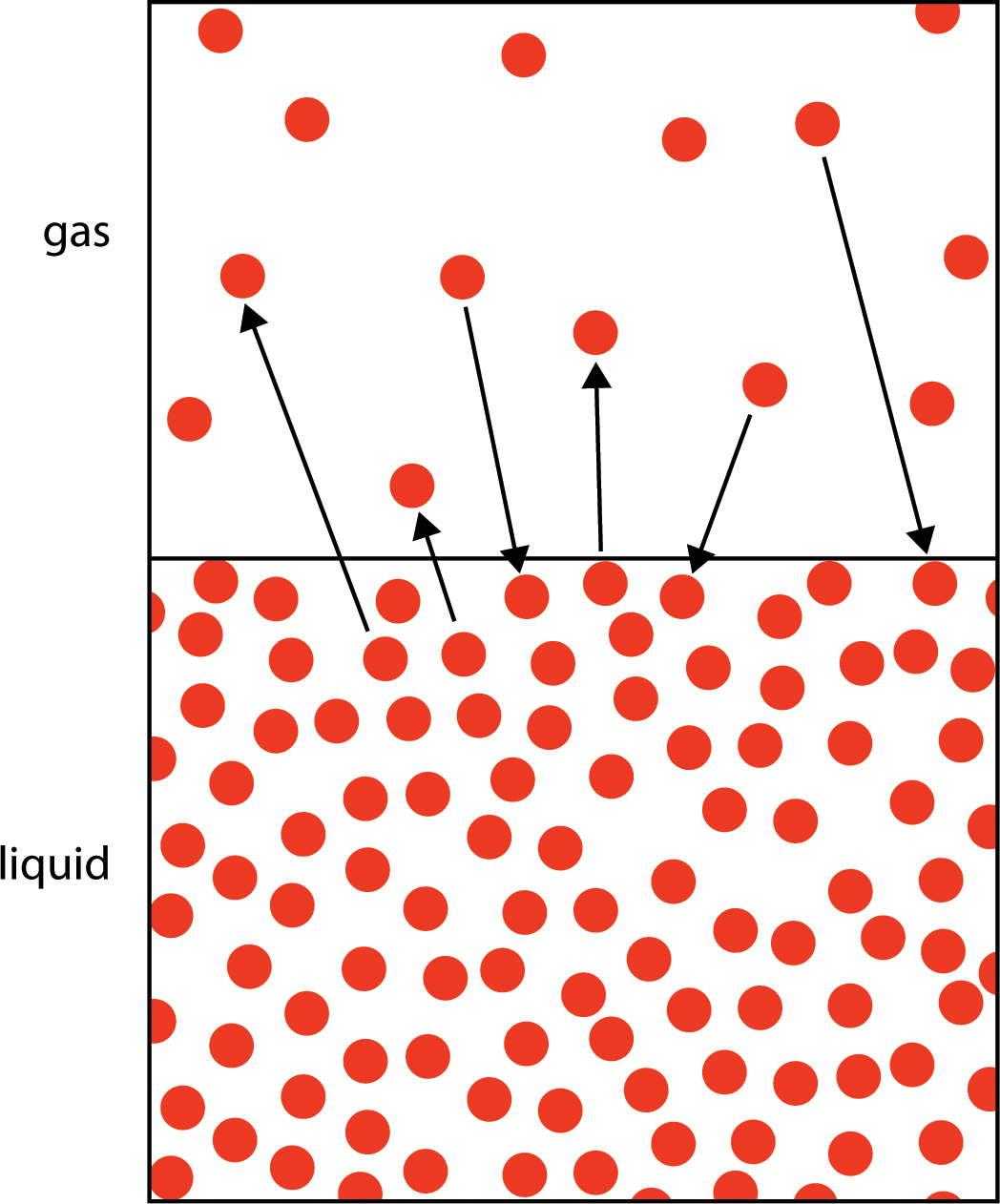
Q What S The Difference Between Evaporation And Boiling Nsta
Why Do Some Liquids Evaporate Faster Than Others Quora

Do All Liquids Evaporate At The Same Rate Science Project Education Com
How Does Water Evaporate Below Its Boiling Point Quora
Question Do All Liquids Evaporate At The Same Rate The Science Shop
Q What S The Difference Between Evaporation And Boiling Nsta

Comments
Post a Comment